Pulmonary Function Testing from infant to adult patients
The EXHALYZER® D offers various lung function tests for infants (>3 kg), children and adult patients. The modular system is easily upgradable and fully compliant with the ATS / ERS recommendations for infant and pediatric pulmonary function testing.
Flow and pressure measurement
FRC / LCI infant measurement (option)
Nitrogen washout FRC module (option)
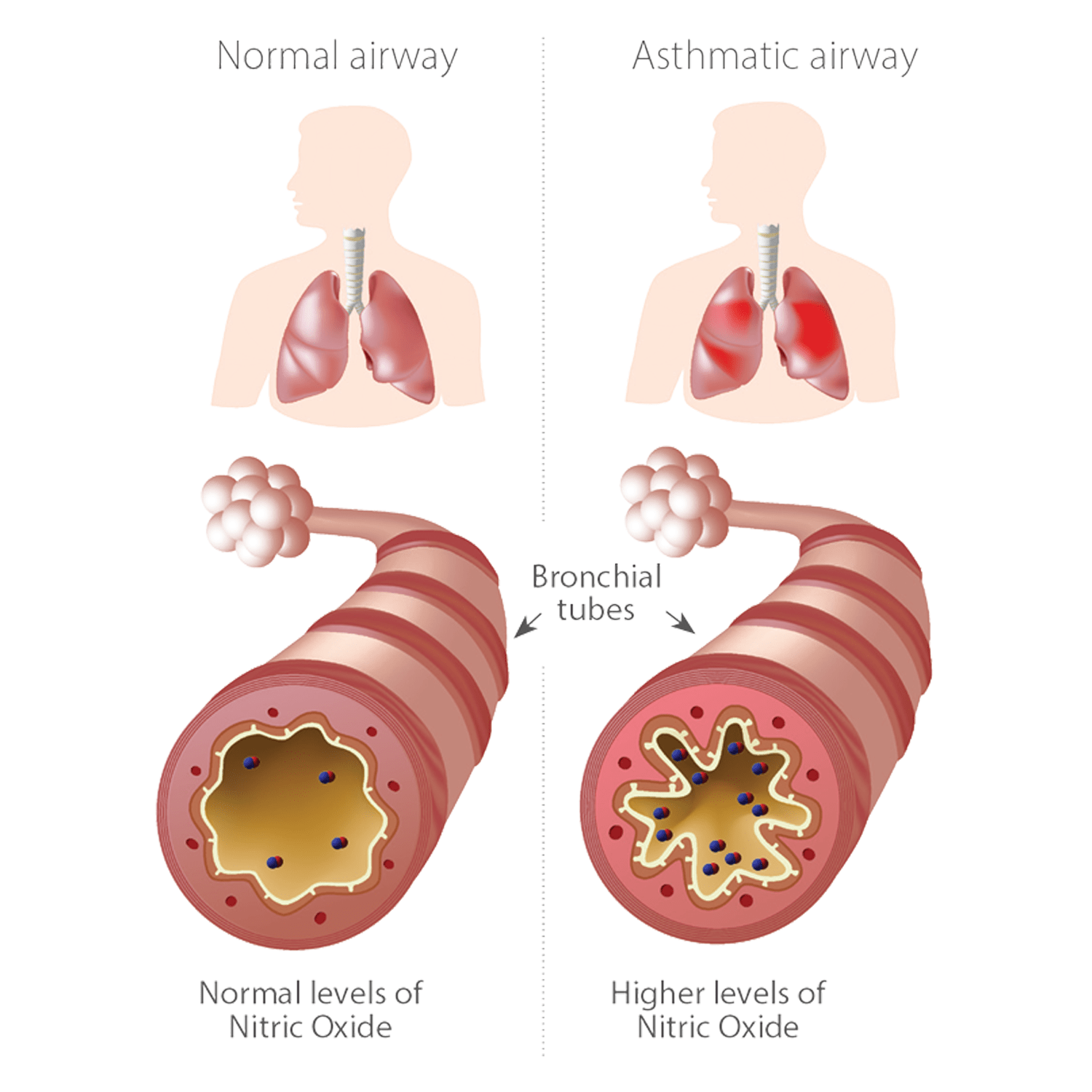
NO measurement (option)
Oxygen measurement (option)
Airway occlusion module (option)
General
The EXHALYZER D offers the “gold standard” in accuracy for clinical application. The EXHALYZER D can be extended with several optional modules. The nitrogen washout bypass system allows the performance of multiple washout tests, while the optional NO measurement module allows the non-invasive detection of lung inflammation (fractional exhaled nitric oxide, FeNO), for example. The instrument works independently of the temperature, humidity or viscosity of the gas.
The instrument and software are fully compliant to the recent ATS / ERS recommendations for pulmonary function testing and exhaled nitric oxide measurements.
Easy operation
All tests are easily started, executed, recorded and evaluated by the SPIROWARE® software. Display options include numerical and graphical data analysis. Respiratory conditions can be monitored over time, from newborn to adult age.
One system for pulmonary function testing for all patients: From infants to school age children to adults, all age groups can be tested on the EXHALYZER D. In particular, the EXHALYZER D is ideally suited for infants (>3 kg). The low airflow restriction prevents interference with the infant’s spontaneous breathing. Furthermore, infant sedation is not required for standard tests. Respiratory conditions can be easily followed up from infants to adults.
Service is essential to keep medical equipment operational and safe. Our Nordic service team consists of experienced service technicians stationed across the Nordic countries. Our service technicians are ready to take on your every service need.”

Meeting the needs of medical staff and patients
The Vivo 55 is an advanced homecare ventilator designed to deliver secure and comfortable treatment to patients from 10 kg. The Vivo 55 can be used for a wide variety of patients, both life-support and non life-support, thanks to a comprehensive set of modes, circuits and accessories.
The Vivo 55 is designed to meet the needs both of medical staff and a wide range of patients. It combines comfortable and controllable ventilation with excellent monitoring capabilities to potentially reduce readmissions to acute care facilities. The extensive monitoring capabilities help obtain a better insight into the quality of ventilation. The Vivo 55 is an excellent choice for mechanical ventilation at home, in the hospital and in long-term care facilities. The Vivo 55 is prepared for connection to Breas cloud solutions.

Versatile Airway Clearance
Introducing the Clearway 2 with TreatRepeat®. The second-generation MI-E device by Breas incorporates the latest technology to bring effective and accurate treatment for patients requiring airway clearance and cough assistance. TreatRepeat function allows clinicians to manually deliver different numbers of insufflations and exsufflations and then save the treatment they have just delivered to the device to be repeated automatically. Helping to improve accuracy and speed of titration and set-up.
Multiple mode options
Up to four treatment profiles can be saved to the device.The settings used in the manual and programmed modes are also saved to the device. This allows for up to 7 MI-E prescriptions to be saved. The treatment settings in the IPPB and NIV modes are also saved. The programmable mode enables the delivery of multiple insufflations as part of a cough cycle.

IOS Impulse Oscillometry
Tidal breathing analysis with Impulse Oscillometry (IOS) has demonstrated to be informative and differentiated in the early detection and follow up of pulmonary diseases like asthma, COPD and idiopathic pulmonary fibrosis. IOS is almost independent of patient cooperation and can test a larger patient range than spirometry alone, from children to adult to geriatric patients.
IOS is available as a stand-alone device combined with a spirometry measurement program (MasterScreen IOS and Vyntus IOS) or as an add-on module to the MasterScreen series.

The dual-valved LiteAire MDI holding chamber: Collapsible, disposable paperboard design.
The LiteAire® collapsible MDI holding chamber is a unique MDI holding chamber and an innovative alternative. LiteAire’s unique dual-valved MDI holding chamber design delivers pop-up convenience and effective drug output at a fraction of the cost of most plastic holding chambers. In many clinical settings, the LiteAire can reduce costs by replacing existing rigid plastic holding chambers or inefficient spacers with a paperboard alternative. The unique design allows the LiteAire to be reused by a patient over multiple doses and meets and often exceeds the performance of plastic holding chambers.